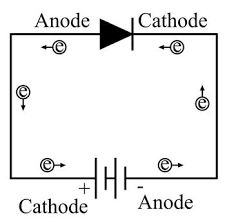
Selection of lithium electronic electrode materials and battery core materials
The internal components of a lithium-ion battery are mainly positive electrode | electrolyte | separator | electrolyte | negative electrode. On this basis, the welding of the tabs, wrapping of the outer packaging and other steps are carried out to form a complete battery core finally. After the battery core goes through the preliminary charging and discharging, development, quantity splitting up and other activities, it can be used before leaving the manufacturing facility. The first step in the process is material selection. The factors affecting materials' safety are mainly their intrinsic orbital energy, crystal structure and material properties.
Cathode material
The main role of the cathode active material in the battery is to contribute specific capacity and specific energy, and its intrinsic electrode potential has a certain impact on safety. For example, in recent years, China has used the medium and low-voltage material LiFePO4 (lithium iron phosphate) as a cathode material for power batteries and has been widely used in transportation (such as hybrid electric vehicles HEV, electric vehicles EV) and energy storage equipment (such as uninterruptible power supplies) UPS), but the safety advantage of LiFePO4 among many materials is actually at the expense of energy density, which restricts the battery life of its users (such as EVs, UPS). Although ternary materials such as NMC (LiNixMnyCo1-x-yO2) perform well in energy density as ideal cathode materials for power batteries, safety issues have not been fully solved.
Researchers have done a lot of work to study the thermal behavior of cathode materials and found that the intrinsic electrode potential and crystal structure are the main factors affecting its safety. For example: whether the potential μC of the cathode material and the highest molecular occupied orbital HOMO of the electrolyte are perfectly matched directly affects the stability of the electrolyte; whether multiple lithium ions can be smoothly passed through the lattice at the same time will lead to reactions between different cathode materials and electrolytes. The starting temperature and heat release are different. For instance, when Li0.5 CoO2 coexists with the electrolyte (LiPF6/EC/DEC), the beginning temperature of the oxygen launch reaction is 130 ° C, while LiFPO4 launches oxygen in the same electrolyte environment. The starting temperature of the thermal reaction is around 190°C, and the heat of the reaction is small. By selecting material types and element doping, selecting materials with potentials that match the electrochemical window of the electrolyte, higher initial reaction temperatures, and less reaction heat can improve the safety performance of the battery core from the perspective of positive active materials.

Negative electrode material
The influence of adverse energetic materials on security efficiency generally originates from the setup relationship between its innate orbital power and the electrolyte LUMO. Throughout fast charging, the speed of lithium ions going through the SEI (solid electrolyte user interface) film might be slower than the deposition speed of lithium on the unfavorable electrode. Li dendrites will continue to grow with the charge and discharge cycle, which may cause internal short circuits and ignite. The flammable electrolyte undergoes thermal runaway, which limits the safety of the negative electrode during fast charging. Only when the potential difference between the negative electrode material and the potential of lithium is less than -0.7 eV, that is, μA<μLi-0.7 eV, can the deposition of lithium not cause a short circuit. For safety reasons, power batteries should use negative electrode materials with a potential μA<1.0 eV (relative to Li+/Li0) to achieve safe, fast charging. During the discharge process, there is no speed competition for lithium ions passing through the SEI film and depositing on the negative electrode, so the fast discharge process is safe. Li4Ti5O12, as shown in Figure 4, has a safety advantage in the field of fast charge and discharge because its potential is 1.5 eV (relative to Li+/Li0), which is lower than the LUMO of the electrolyte. There is also a new negative electrode material, Ti0.9Nb0.1Nb2O7, which can be rapidly charged and discharged for more than 30 weeks at a voltage of 1.3 to 1.6 V (relative to Li+/Li0), and has a specific capacity of 300 mA·h/g, which is higher than LTO.
In addition to the growth of lithium dendrites, the reaction between the anode material and the electrolyte is an important factor affecting safety performance. Around 100 ° C, the exothermic top of lithium-embedded graphite and electrolyte can be observed, which is likewise thought to be the SEI movie's decay response. The reaction rate increases as the specific surface area of the negative electrode material increases. After the SEI film is decomposed, the lithium embedded in the negative electrode will continue to react with the electrolyte and binder to release heat. The reaction heat increases as the amount of lithium embedded increases. By improving the thermal security of SEI, decreasing the details area of the adverse electrode material, and lowering the quantity of lithium ingrained, the efficiency of the battery core can likewise be improved from the perspective of the adverse electrode product.
Electrolytes and separators
The main impact of electrolytes and separators on safety is their properties.
Although the thermal stability of lithium salt is the fundamental factor affecting the thermal stability of the electrolyte, its impact on battery safety performance is limited because its decomposition reaction heat is small. The flammability and liquid state of currently widely used commercial electrolytes are important safety factors. If a solid electrolyte with a lithium-ion conductivity σLi+>10-4 S/cm is used, on the one hand, it can prevent lithium dendrites from piercing the separator and reaching the positive electrode to solve the safety problem; on the other hand, it can solve the problem of the contact between the negative electrode and the carbonate electrolyte and the positive electrode. Thermal stability issues arising from contact with aqueous electrolytes. In addition, using electrolytes with a wider electrochemical window (especially higher LUMO), adding flame retardant materials to the electrolyte, such as mixing ionic liquids and organic liquid electrolytes to modify them into non-flammable electrolytes, etc., can all improve safety. Effective means of sex.
The separator's mechanical strength (tensile and puncture strength), porosity, thickness uniformity and interruption/rupture temperature are important factors in determining its safety. The application of ceramic coatings in separators can increase the mechanical strength of the original film, allowing the separator to exhibit excellent performance in terms of high-temperature resistance, puncture resistance, and thickness reduction. At the same time, to ensure the safety of the battery core, the porosity of the separator should generally be less than 50%, and the thickness should be more than 20 μm. If the temperature at which the microporous structure closes is too high or too low, it will affect the performance of the battery core. Therefore, the separator polymer's composition and the porous structure's optimal configuration need to be comprehensively considered. At the same time, the rupture temperature should be higher than the interruption temperature.

High Quality Silicon-Carbon Material Anode Supplier
Graphite-crop corporate HQ, founded on October 17, 2008, is a high-tech enterprise committed to the research and development, production, processing, sales and technical services of lithium ion battery anode materials. After more than 10 years of development, the company has gradually developed into a diversified product structure with natural graphite, artificial graphite, composite graphite, intermediate phase and other negative materials (silicon carbon materials, etc.). The products are widely used in high-end lithium ion digital, power and energy storage batteries.If you are looking for Silicon-Carbon Anode Material,click on the needed products and send us an inquiry: sales@graphite-corp.com







