Essential information for lithium batteries: charge and discharge curve analysis
The charge and discharge curve reflects the charge and discharge behavior of the lithium battery material. The analysis of the button battery charge and discharge curve is of great significance to knowing the performance and electrochemical behavior of the material. In particular, the half-cell charge and discharge curve analysis can provide targeted analysis—the characteristic behavior of a certain material. There are several different presentation forms of charge and discharge curves, such as the more common "cross-type" curve (Figure 1) and the "cyclic" curve (Figure 2).
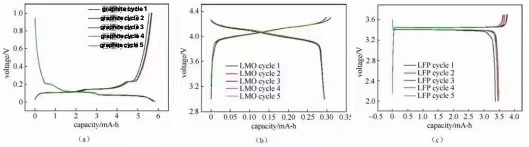
Figure 1: "Crossover" charge and discharge curves of half cells assembled from several different materials
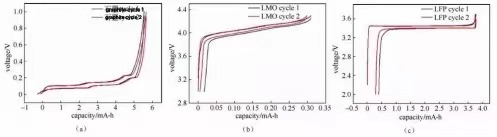
Figure 2: “Cyclic” charge and discharge curves of half-cells assembled from several different materials
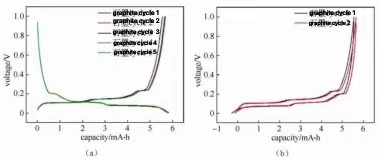
Figure 3: Charging and discharging curves of graphite/lithium metal plate button half-cell
A large quantity of data info can be checked out from the switch battery cost and discharge contour. The following briefly introduces the analysis and evaluation of some information.
The deintercalation of lithium ions in the positive and adverse electrode materials represents the plateau or slope location on the fee and discharge contour (along with the redox height in the cyclic voltammetry curve and differential resistance contour). The materials can be analyzed and studied according to the changes in each plateau location—electrochemical response behavior. The number of potential systems or inclines for charging and discharging is the same. Suppose the overall capability of charging and discharging is the same. However, the capability of each matching platform/slope is different; it suggests the material's thermodynamic response course or the lithium intercalation and desorption power. There are considerable differences in scholastic characteristics. Figure 3 shows the fee and discharge contours of normal graphite anode products. The charge and discharge curve reveals that when the graphite/metal lithium sheet half-cell is billed and released, there are 3 matching apparent charge and discharge platforms at 0.08/ 0.1 V, 0.11/ 0.14 V and 0.2/ 0.22 V during the graphite electrode charge and discharge procedure, specifically. The two-phase improvement process of three lithium-graphite interlayer compounds was studied. The starting factor of the platform represents the beginning of the phase modification, and the completion factor corresponds to the completion factor of the stage adjustment. The system habits indicate that the host material's electrochemical potential has nothing to do with the occupancy price of ions in the product. The incline in the charge-discharge curve typically represents a strong option response or capacitive habits. The slope habits mean that the host product's electrochemical possibility is straight to the occupancy price of ions in the product.

For that reason, the charge and discharge curves can be made use of to determine the number of stages preliminarily transform the product has throughout the response procedure, whether it is a two-phase change response or a solid option, adsorption and desorption capacitance habits, which can aid in a leading architectural research study such as X-ray diffraction. Under the very same SOC, when charging and discharging with a small present, the intermediate worth of the charging prospective platform and the discharge potential platform voltage is roughly the thermodynamic balance capacity. The intermediate possible value of the oxidation top and decreased height corresponding to the cyclic voltammetry curve or differential capability curve is utilized. It is easier to approximate accurately. For exact thermodynamic stability potential dimension at complete SOC, it is suggested to make use of the GITT approach at reduced present thickness.
In the complete battery discharge habits, the discharge voltage of the battery is the lithium insertion voltage of the desirable electrode product minus the delithiation voltage of the adverse electrode material.
Therefore, the higher the ordinary delithiation platform of the unfavorable electrode, the reduced the discharge voltage of the complete battery. When the delithiation platform of the anode product goes beyond 2.0 V, the complete battery voltage is already really low. The measured ability currently is of little significance to complete battery matching and useful applications because each electrical application has a permitted lower limitation voltage range. The discharge voltage of lithium-ion batteries commonly used in customer electronic appliances is reduced to 2.7 V.
Energy performance is the proportion of discharge power to charge energy in the same cycle, which can be revealed as η = (EDQD) (/ ECQC) × 100%. The charge-discharge curve can be estimated by the incorporated location difference of the charge-discharge curve, and the modification in this worth is less complicated to read in the "cyclic" charge-discharge curve. The power effectiveness of common lithium-ion batteries is 92% to 95%, while the energy efficiency of lithium-sulfur and lithium-air batteries is around 80% and 70%, respectively.
By evaluating the initial five weeks' cost and discharge cycle data, information such as first week discharge capacity, initial week charging capacity, very first week Coulomb efficiency, relatively easy to fix ability, polarization voltage and resistance size, energy performance and other info can be acquired.
The cost and discharge data in the initial week are the most essential. The discharge ability in the very first cycle can be checked straight from the contour and used to examine the actual discharge capacity of the post-item after the initial cycle. The cost and discharge platform of the battery in the first week lays the structure for subsequent cycles. The stability of the structure of many materials is also figured out by the first week. The system's size likewise impacts the insertion and removal efficiency of lithium ions. The cost and discharge ability in the 2nd and succeeding weeks fluctuate based on the discharge capacity in the first week. Coulombic effectiveness (i.e., fee and discharge effectiveness) describes the proportion of battery discharge ability to billing capacity throughout the same cycle, eta = QD/QC × 100%. Coulombic effectiveness (i.e., very first result) in the very first week is the battery's efficiency. The proportion of discharge capability to charging capacity (favorable electrode material η=QD1/QC1 × 100%). Numerous battery testing systems can straightly output this value, which can make use of analyze the capacity of the pole piece taken in by activation and various other responses throughout the first cycle and can directly define the stability of the product framework and the quality of the vibrant performance.
The first discharge capacity and first-cycle Coulomb effectiveness can directly influence the layout of the full battery and the examination of materials. The Coulombic effectiveness in the first 5 weeks typically shows a fad at first rising and afterward decreasing or fluctuating slightly. This results from the permanent loss of active lithium source triggered by responses such as SEI film growth and product activation during the very first couple of weeks of biking. Taking a half-cell with fresh adverse electrode material as an example, its initial discharge capacity is greater than its very first charge capability; that is, the initial lithium insertion amount of the unfavorable electrode is above the very first lithium elimination quantity of the adverse electrode. If the examination result is contrary, it may result from non-fresh pole items or battery short circuit variables.

The optimum ability, that is, the highest possible capacity value presented during the fee and discharge procedure of the test battery, normally takes place during the initial 5 weeks of cost and discharge. Examination outcomes of some anode materials reveal that the reversible ability continues to enhance as the variety of cycles increases. This relates to the ongoing oxidation and sluggish activation of the material, the ongoing development of the SEI movie, and the gradual involvement of other materials in the oxidation reaction. This kind of anode material is a downside as opposed to an advantage for the style and application of lithium-ion full batteries. Typically speaking, the relatively easy to fix capacity measured by the battery will likely be reasonably steady in the initial 5 weeks. The Coulombic efficiency can not reach 99.95% quickly, which indicates that the user interface or material structure has been unpredictable. Compared with half-cell testing, such materials are utilized for full-cell testing. The cyclicity will certainly be much worse.
Supplier
Graphite-crop corporate HQ is a high-tech enterprise committed to the research and development, production, processing, sales and technical services of Silicon materials, we are high quality silicon supplier. Our Company has a diversified product structure with silicon carbon materials, and other negative materials (graphite materials, etc.). Please feel free to contact us. Or click on the needed products and send us an inquiry: sales@graphite-corp.com.







