Attenuation effects in super-fast charging of lithium-ion power batteries
Temperature effect
The heat generation of lithium-ion batteries can be divided into reversible and irreversible processes. The expression of non-thermogenic Qirr is as follows:
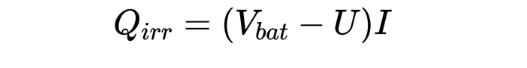
U is the open circuit voltage, Vbat is the battery voltage, and I is the current (when charging). Most of the irreversible heat comes from internal resistance heat generation:
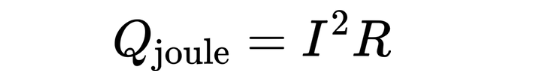
Among them, R is the internal resistance of the battery. Joule heat is proportional to the square of the current, so as the current increases during fast charging, the irreversible heat will increase significantly. The reversible heat Qrev comes from the entropy change in the electrochemical reaction, also known as entropic heat. Its expression is:
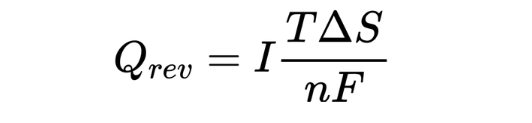
In lithium-ion batteries, the heat distribution and dissipation of soft-pack, cylindrical, and square-shell batteries are unevenly distributed. For example, some battery materials have poor surface thermal conductivity, so their heat will accumulate more in the core than on the surface. The current density and heat generation rate are also different at different locations in the battery. These inconsistencies are further amplified on larger cells. As shown in Figure 3, the temperature in the center of the cylindrical battery is significantly higher than the surface. For soft-pack or square batteries, as shown in Figures 4 and 5, the temperature at the tabs is significantly higher than at other locations. In addition, since the resistance of the positive aluminum current collector is greater than that of the negative copper current collector, the temperature of the positive electrode tab is often higher than that of the negative electrode tab.
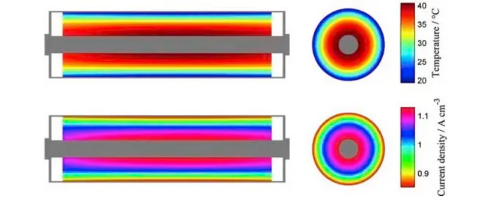
Figure 3: Simulation results of distribution of temperature and current density inside cylindrical battery
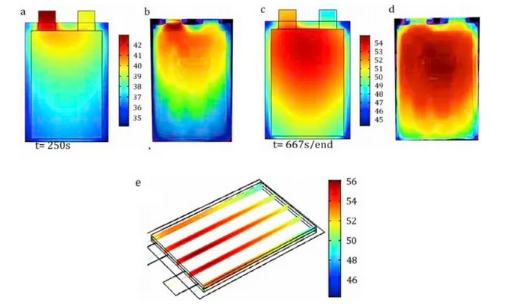
Figure 4: Surface temperature changes of soft-pack batteries during constant current discharge at 5C: a) simulation results and b) measurement results for t=250s; c) simulation results and d) measurement results for t=667s; e) internal temperature 3D distribution
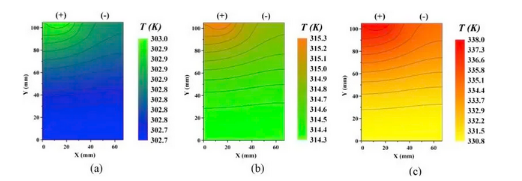
Figure 5: Temperature distribution diagram of LFP battery discharged at a) 1C, b) 2C and c) 5C
Uneven distribution of heat production not only exists in battery cells but likewise calls for focus on the layout of the thermal management system at the battery pack degree because it has a considerable effect on the temperature level circulation within the Pack. As time goes by, the different aging paths of the battery cells will also greatly impact the uniformity of heat production of the Pack. This is due to the different increases in the internal resistance of different batteries. To solve this problem, the design of the thermal management system will be introduced in Section 6.
Many aging mechanisms in lithium-ion batteries are temperature-related. At high temperatures, the SEI film accelerates growth on the negative electrode and becomes more loose and unstable. At low temperatures, ion diffusion and reaction rates slow down, and the possibility of lithium evolution and lithium dendrite growth increases. Almost all aging reactions will be accelerated at high temperatures; low temperatures can reduce the side reaction rate and the diffusion of active materials. If lithium metal precipitates, the decay will be accelerated. In addition, increased low-temperature polarization will lead to increased heat production and reduced energy efficiency. Under most operating conditions, the main attenuation mechanism is SEI film growth at the anode/electrolyte interface. The SEI film will increase the battery's internal resistance and reduce the power, leading to capacity fading. At high temperatures (60°C or higher), SEI components will dissolve and decompose, destroying the integrity of the negative electrode protective film. Extreme thermal runaway may occur when battery temperature exceeds safety thresholds.
Impact of Lithium Precipitation
Lithium evolution refers to the Faradaic side reaction in which lithium ions in the electrolyte are deposited as lithium metal on the negative electrode rather than the process of embedding into the negative electrode particles. Lithium evolution may occur when the negative electrode potential drops under Li/Li+. Lithium metal will first form droplets during the lithium precipitation process to reduce the surface energy. The surface metal and the electrolyte react quickly to form an SEI film. As even more lithium is deposited below the SEI film until the SEI film ruptures, a brand-new SEI movie is formed on the lithium surface, the lithium salt concentration gradually lowers, and lithium metal begins to grow perpendicular to the surface of the pole item, creating lithium dendrites. Lithium dendrite development is thought to be among the worst side reactions. If the dendrites penetrate the separator and reach the positive electrode, an internal short circuit will cause the battery to generate heat rapidly. Lithium metal is more active than the negative electrode, which further causes internal side reactions, leading to problems such as SEI growth, gas production, and electrolyte dissolution.
Researchers have proposed some models for lithium precipitation observations, including the lithium evolution model based on the P2D model by Fuller, Doyle and Newman and the reversible lithium embedding process proposed by Arora, Doyle and White. On this basis, Perkins proposed a control-oriented reduced-order model; Hein and Latz proposed a three-dimensional microstructure analytical model. Ren also considered the intercalation of reversible lithium and the reaction of irreversible lithium (dead lithium) during the battery charging.
Non-destructive lithium evolution observation technology is important for practical battery applications. Tests usually used to characterize lithium evolution include SEM, TEM, NMR and XRD. Yet, these techniques call for the destruction of the battery or the use of special battery setups. Typically used non-destructive lithium analysis monitorings utilize the exterior attributes of the battery, including aging rate, voltage platform for lithium back-intercalation, model prediction and other methods. As shown in Figure 6, lithium evolution detection methods based on aging characteristics include (a) Arrhenius equation, (b) analysis of capacity and impedance changes in the attenuation process, (c) nonlinear frequency domain response analysis and (d) Coulomb efficiency analysis.
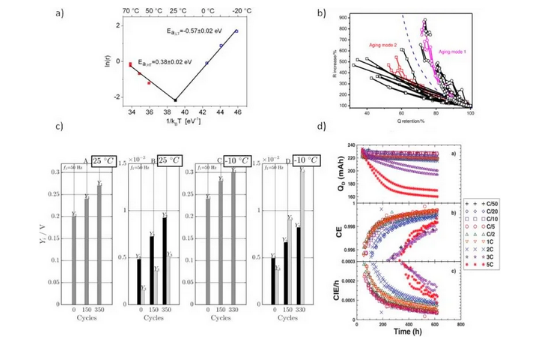
Figure 6: Lithium evolution detection method based on aging characteristics
Part of the lithium released will be re-embedded in the negative electrode or be eluted during the discharge process. The relaxation process after charging or the subsequent discharge process will generate a new voltage platform, as shown in Figure 7. Voltage differentiation (DVA) and capacity differentiation (ICA) help find voltage platforms. Still, these methods require a small discharge rate, and large currents will increase polarization and cover the lithium evolution signal on the voltage curve. Lithium precipitation and re-intercalation may also cause abnormal heat generation peaks as one of the signals of lithium precipitation. The increase in battery thickness may also lead to lithium precipitation, but the relevant mechanism requires further study. Predicting lithium evolution using electrochemical models often depends on charging conditions. However, these models are too complex and require a lot of calculations, and they need to be further simplified to achieve online detection. A few methods can achieve quantitative in-situ detection of lithium evolution after abnormal battery charging. The author believes that the detection method based on the abnormal voltage platform is the most promising for application. However, there are still high knowledge and technical barriers to its application.

Figure 7: Lithium evolution detection based on lithium back-intercalation
a) Simulation of overpotential changes during CC-CV charging and standing. Stage I, there is no lithium deposition on the negative electrode particles; Stage II, lithium deposition begins to occur; Stage III, part of the reversible lithium is re-embedded in the negative electrode or dissolved, and the rest becomes dead lithium; Stage IV, equilibrium state, dead lithium no longer participates in subsequent processes Cycle;
b) Differential Voltage Analysis (DVA);
c) Differential Capacity Analysis (ICA)
High quality lithium-ion batteries supplier
Graphite-crop corporate HQ, founded on October 17, 2008, is a high-tech enterprise committed to the research and development, production, processing, sales and technical services of lithium ion battery anode materials. After more than 10 years of development, the company has gradually developed into a diversified product structure with natural graphite, artificial graphite, composite graphite, intermediate phase and other negative materials (silicon carbon materials, etc.). The products are widely used in high-end lithium ion digital, power and energy storage batteries.If you are looking for Lithium battery anode material,click on the needed products and send us an inquiry:sales@graphite-corp.com







