Professional graphite material supplier, graphite for EV, grease, furnace and any other industries.
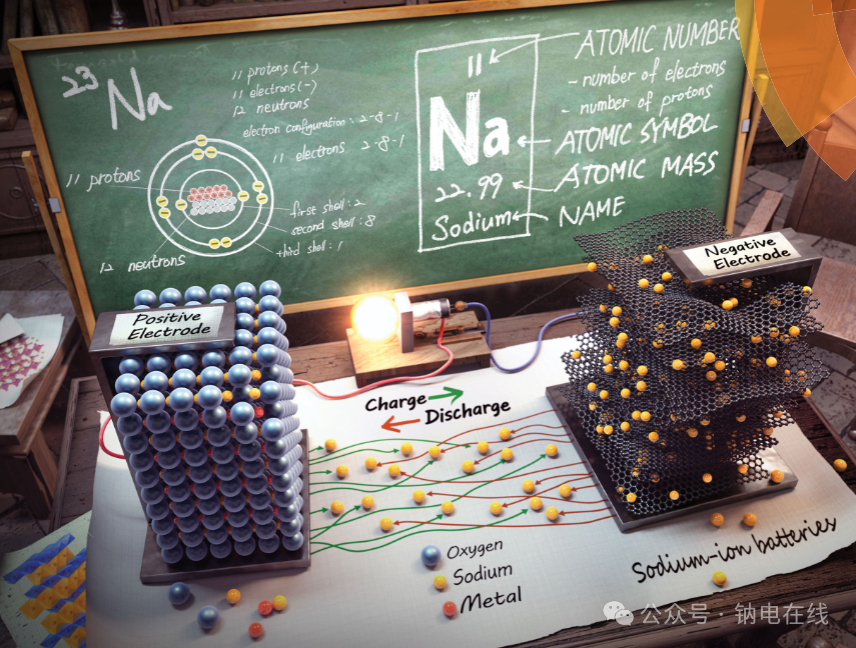
Advances in electrode material research:
Cathode materials include two-dimensional layered transition metal oxides (O3 type, P2 type, etc.), three-dimensional layered transition metal oxides and fluorides, polyanionic compounds (phosphates, pyrophosphates, NASICON type, etc.), cyanides, and organic compounds. Different types of materials have distinct characteristics in terms of crystal structure, sodium ion storage mechanisms (intercalation, conversion reactions, etc.), capacity, voltage, and cycling stability. For example, layered oxides offer high capacity but require optimization for improved cycling stability, while polyanionic compounds exhibit excellent thermal stability but suffer from poor conductivity.
Anode materials include carbonaceous materials based on intercalation reactions (graphite, hard carbon, graphene, etc.), titanium-based oxides, transition metal oxides/sulfides/phosphides based on conversion reactions, Group 14 (Si, Ge, Sn) and Group 15 (P, Sb, Bi) elements, and binary intermetallic compounds based on alloying reactions, and organic compounds. Hard carbon is a mainstream candidate due to its low potential and long cycle stability, while conversion-type and alloying-type materials offer higher capacity but face challenges such as large volume expansion and sluggish kinetics.
Electrolytes, additives, and binders
Electrolytes mainly consist of organic electrolytes with carbonate esters as solvents and NaPF₆ or NaClO₄ as salts. Ether-based electrolytes are also gradually attracting attention, and aqueous electrolytes have potential application value due to their low cost and high safety.
Additives (such as fluoroethylene carbonate, FEC) can improve battery performance by forming a stable solid electrolyte interphase (SEI). Regarding binders, water-soluble sodium carboxymethyl cellulose (Na-CMC) and polyacrylic acid (PAA) offer environmental advantages compared to traditional PVDF, and can mitigate structural damage caused by electrode volume changes.
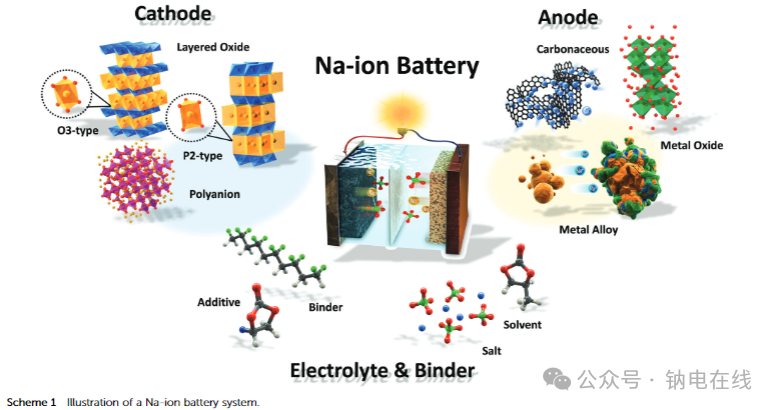
Sodium-ion battery design and challenges
Full-cell research has explored various combinations of cathode and anode materials, with some systems exhibiting high operating voltages (such as the 4.0 V range) and cycling stability. However, challenges remain regarding capacity balance, voltage range matching, and electrolyte stability.
The core challenges facing sodium-ion batteries include: the large radius of sodium ions leading to significant structural changes in electrode materials, the strong hygroscopicity of some materials, electrode volume expansion and contraction, poor kinetic performance, and the need for material consistency and cost control for large-scale production.
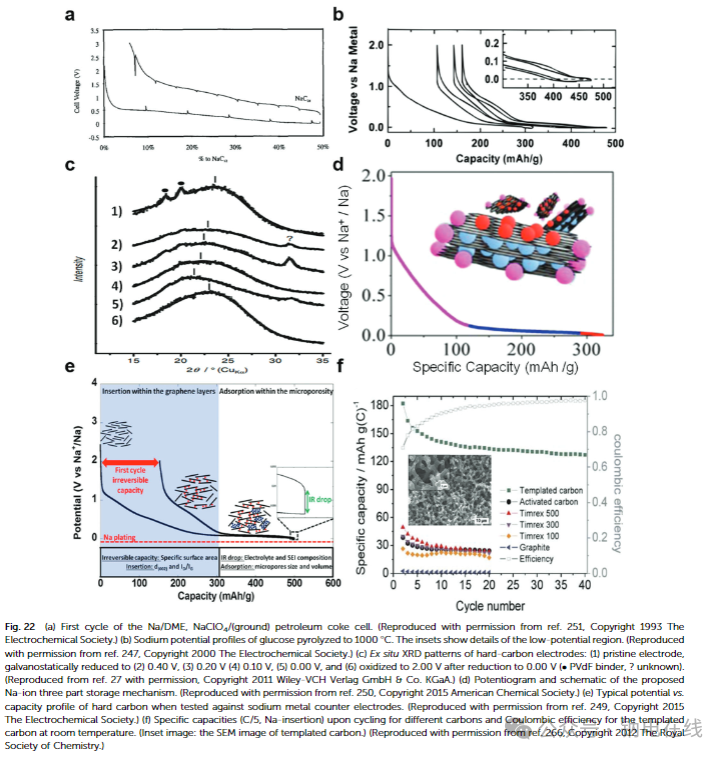
Sodium-ion batteries, as a potential alternative to lithium-ion batteries, possess broad application prospects in areas such as large-scale energy storage due to their core advantages of abundant sodium resources and low cost. This review comprehensively summarizes the current research status of materials for sodium-ion batteries. Regarding cathode materials, layered transition metal oxides and polyanionic compounds each have their advantages and disadvantages. O3-type layered materials have greater practical potential due to their structural similarity to commercial lithium-ion battery cathodes, while P2-type materials, although having higher capacity, require addressing the issue of initial cycle Coulombic efficiency. Among anode materials, hard carbon remains the mainstream choice due to its low potential and long cycle stability, while high-capacity conversion-type and alloying-type materials require structural design to mitigate volume expansion issues. Optimization of electrolytes, additives, and binders is crucial for improving battery performance; suitable electrolyte formulations can reduce interfacial reactions, additives such as FEC can improve SEI film stability, and water-soluble binders are more environmentally friendly and cost-effective.
Although significant progress has been made in the material development and battery design of sodium-ion batteries, they still face multiple challenges, including material structural stability, ion transport kinetics, control of electrode volume changes, and large-scale production. Future efforts need to focus on further optimizing the crystal structure and surface modification of electrode materials, improving the capacity balance and voltage matching design of full cells, developing high-performance electrolyte systems, and promoting collaborative innovation in computational chemistry, material synthesis, and characterization techniques to achieve the commercial application of sodium-ion batteries in practical energy storage scenarios.






