Professional graphite material supplier, graphite for EV, grease, furnace and any other industries.
From Graphite Mine to Battery Anode: A Global Journey
(From Graphite Mine to Battery Anode: A Global Journey)
Think about the sleek electric car silently gliding down the road. Or the powerful laptop keeping you connected. Or the handy cordless drill building your latest project. What gives these devices their energy? Often, it’s a lithium-ion battery. And a key player inside that battery is something called the anode. Our journey today starts deep underground and ends up powering our modern lives. Let’s trace the fascinating path of graphite becoming a vital battery anode.
What Exactly is a Battery Anode?
Inside a battery, chemical reactions create electricity. These reactions happen between two main parts: the cathode and the anode. Think of the anode as the battery’s workhorse. During discharge, when the battery powers your device, lithium ions flow out of the anode and move towards the cathode. This movement of ions creates the electric current you use. When charging, the process reverses; lithium ions flow back into the anode, getting stored for later use. So, the anode is crucial. It needs to hold lots of lithium ions efficiently and reliably, charge after charge. This is where graphite shines.
Why is Graphite Used in Lithium-ion Batteries?
Graphite isn’t just for pencils anymore. It’s the superstar material for most lithium-ion battery anodes today. Several reasons make it ideal. Graphite has a layered structure. Think of it like stacks of very thin sheets. Lithium ions can easily slip between these sheets. This allows graphite to store a significant amount of lithium. This storage capacity translates directly to battery energy density – how much power a battery can hold for its size and weight. Graphite is also stable. It doesn’t easily break down or react badly with the battery’s other parts. This stability is key for safety and long battery life. Crucially, graphite conducts electricity well. This allows the electrons released during the lithium movement to flow easily, creating the current we need. Compared to other materials tried over the years, graphite offers the best combination of performance, safety, cost, and reliability for mass-produced batteries.
How Does Graphite Go From Rock to Refined Anode Material?
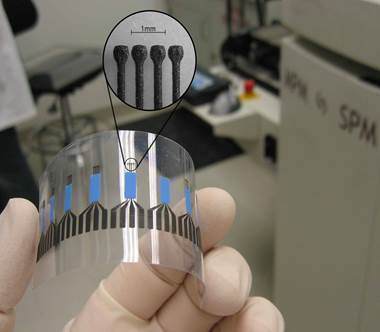
The transformation from raw mineral to high-tech battery component is complex. It starts in mines worldwide. Places like China, Brazil, Mozambique, Madagascar, and Canada produce natural graphite. Miners extract graphite ore, which contains graphite flakes mixed with rock. This ore gets crushed and ground. Then, through processes like flotation, the valuable graphite is separated from the waste rock. But raw, mined graphite isn’t ready for batteries. It needs purification. This often involves heating it to extremely high temperatures or treating it with chemicals. This removes impurities that could harm battery performance. The next critical step is shaping. Battery makers need the graphite particles to be just right. They use processes like spheroidization. This rounds off the sharp edges of graphite flakes, making them more uniform spheres. Why? This shape helps lithium ions move in and out more smoothly. It also allows the graphite particles to pack together tightly. This dense packing means more active material fits into the anode space, boosting the battery’s capacity. Finally, this processed graphite is mixed with binders and solvents to form a slurry. This slurry gets coated thinly onto copper foil. Once dried, this coated foil becomes the finished anode sheet ready for battery assembly.
Where Are Graphite Anodes Used?
Graphite anodes are everywhere lithium-ion batteries are found. And that’s almost everywhere! The biggest consumer is electric vehicles (EVs). Every Tesla, Nissan Leaf, or BYD on the road relies heavily on graphite anodes. The push for longer driving ranges demands high-capacity batteries, making graphite essential. Portable electronics are another huge market. Your smartphone, tablet, laptop, and wireless headphones all contain small lithium-ion batteries with graphite anodes. Power tools benefit too. Cordless drills, saws, and garden equipment use these batteries for portable power. Energy storage is a rapidly growing area. Home batteries storing solar power and large grid-scale storage systems often use lithium-ion technology with graphite anodes. Even medical devices, like some portable monitors, utilize these batteries. Essentially, any device needing rechargeable, lightweight, and relatively high-energy power likely has a graphite anode inside its battery.
Frequently Asked Questions About Graphite Anodes
People often have questions about this key battery component. Let’s tackle a few common ones.
What happens to old graphite anodes? Can they be recycled? Yes, recycling is possible and increasingly important. Old batteries are collected. They undergo processing to recover valuable materials. The graphite itself can be separated. This recovered graphite might be reused in new batteries or find applications in other industries. Recycling helps reduce waste and the need for constant new mining.
Is there enough graphite for all these batteries? Demand is soaring thanks to the EV boom. This puts pressure on graphite supplies. Concerns exist about long-term availability and the environmental impact of mining. Efforts are underway to find more deposits, improve mining efficiency, boost recycling rates, and explore alternative anode materials.
Are there alternatives to graphite? Research is intense. Silicon is a promising candidate. It can potentially store much more lithium than graphite. But silicon has problems. It swells a lot during charging, which can damage the battery. Scientists are working on silicon-graphite blends or new forms of silicon to overcome this. For now, graphite remains the dominant, reliable choice.
Why not use artificial graphite instead of mined? Synthetic graphite is made from petroleum coke or coal tar pitch. It’s processed at super high temperatures. It’s very pure and consistent. But making it uses huge amounts of energy. This makes it more expensive than natural graphite. Natural graphite is generally cheaper, but needs more processing to reach battery-grade purity. Battery makers often use a mix of both types to balance cost and performance.
(From Graphite Mine to Battery Anode: A Global Journey)
How does the graphite anode affect battery life? The anode plays a big role. Over many charge cycles, tiny changes can happen in the graphite structure. Lithium ions might get trapped or side reactions can occur. These degrade the anode’s ability to hold lithium over time. High-quality, well-processed graphite helps maximize battery lifespan. Charging habits also matter; fast charging and deep discharges stress the anode more.